Congratulations to CAI HDR students Sue Lawrence and Ashley Stewart whose publications were selected as joint winners of Round 4 of the CAI 2021 HDR publication competition.
Lawrence, S., Llewellyn, S., Hunt, H., Cowin, G., Sturgess, D., and Reutens, D. "Anatomy of the lumbar interspinous ligament: findings relevant to epidural insertion using loss of resistance." Regional Anesthesia & Pain Medicine, (2021), 46:1085-1090. DOI: 10.1136/rapm-2021-103014
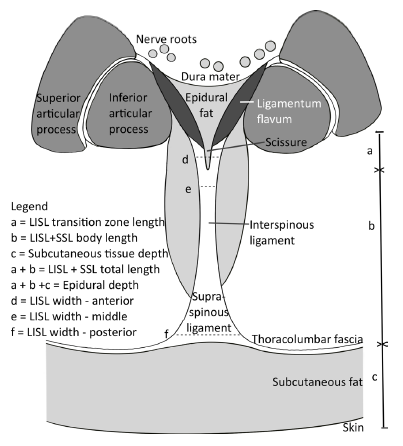
ligament.
ABSTRACT
Background and objectives
The ‘loss of resistance’ technique is used to determine entry into the epidural space, often by a midline needle in the interspinous ligament before the ligamentum flavum. Anatomical explanations for loss of resistance without entry into the epidural space are lacking. This investigation aimed to improve morphometric characterization of the lumbar interspinous ligament by observation and measurement at dissection and from MRI.
Methods
Measurements were made on 14 embalmed donor lumbar spines (T12 to S1) imaged with MRI and then dissected along a tilted axial plane aligned with the lumbar interspace.
Results
In 73 interspaces, median (IQR) lumbar interspinous plus supraspinous ligament length was 29.7 mm (25.5–33.4). Posterior width was 9.2 mm (7.7, 11.9), with narrowing in the middle (4.5 mm (3.0, 6.8)) and an anterior width of 7.3 mm (5.7, 9.8).
Fat-filled gaps were present within 55 (75%). Of 51 anterior gaps, 49 (67%) were related to the ligamenta flava junction. Median (IQR) gap length and width were 3.5 mm (2.5, 5.1) and 1.1 mm (0.9, 1.7).
Detection of gaps with MRI had 100% sensitivity (95% CI 93.5 to 100), 94.4% specificity (72.7, 99.9), 98.2% (90.4, 100) positive predictive value and 100% (80.5, 100) negative predictive value against dissection as the gold standard.
Conclusions
The lumbar interspinous ligament plus supraspinous ligament are biconcave axially. It commonly has fat-filled gaps, particularly anteriorly. These anatomical features may form the anatomical basis for false or equivocal loss of resistance.
Stewart, A.W., Robinson, S. D., O’Brien, K., Jin, J., Widhalm, G., Hangel, G., Walls, A., Goodwin, J., Eckstein, K., Tourell, M., Morgan, C., Narayanan, A., Barth, M., & Bollmann, S. "QSMxT: Robust masking and artifact reduction for quantitative susceptibility mapping." Magnetic Resonance in Medicine, (2021). DOI: 10.1002/mrm.29048
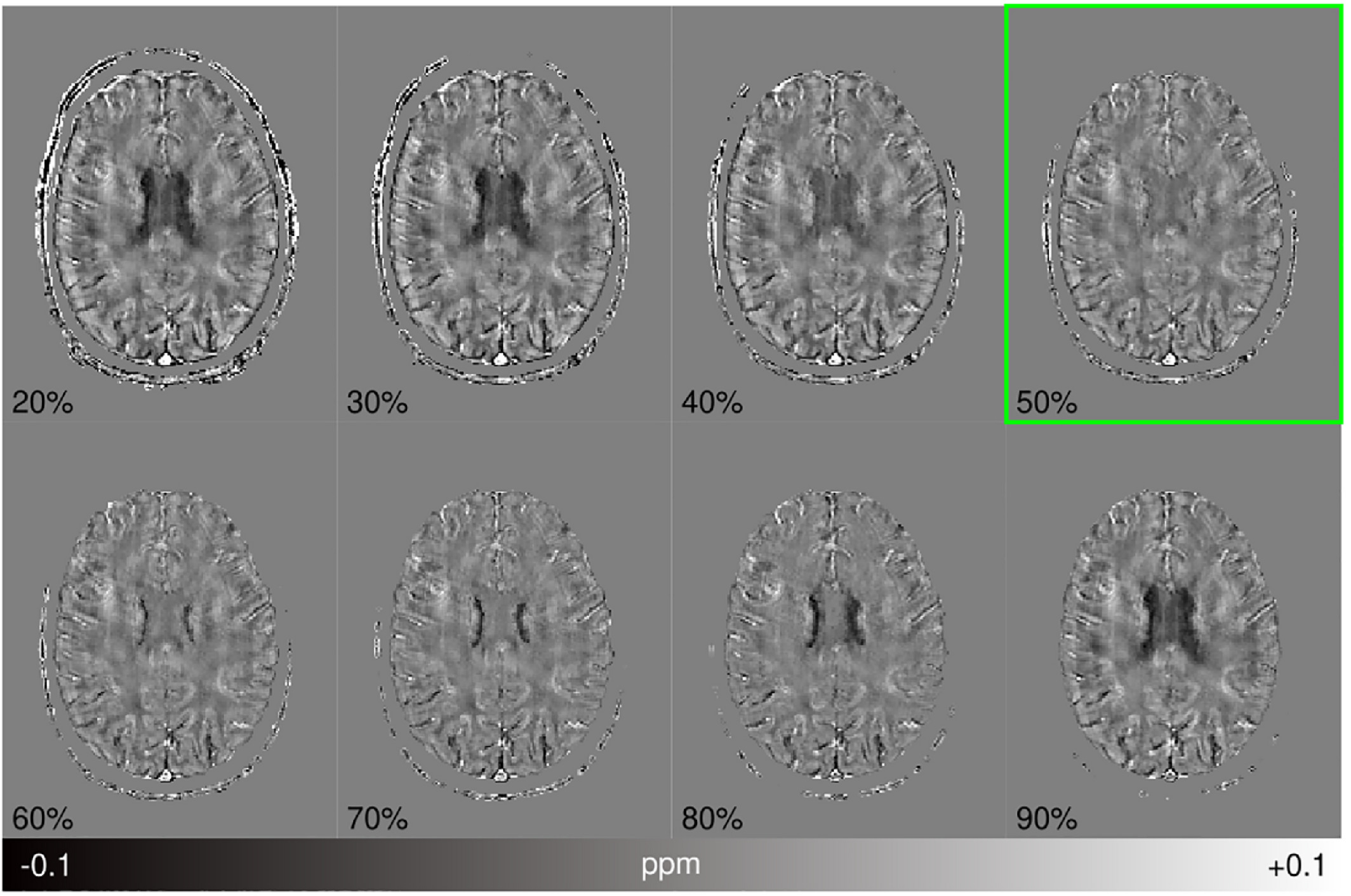
ABSTRACT
Purpose
Quantitative susceptibility mapping (QSM) estimates the spatial distribution of tissue magnetic susceptibilities from the phase of a gradient-echo signal. QSM algorithms require a signal mask to delineate regions with reliable phase for subsequent susceptibility estimation. Existing masking techniques used in QSM have limitations that introduce artifacts, exclude anatomical detail, and rely on parameter tuning and anatomical priors that narrow their application. Here, a robust masking and reconstruction procedure is presented to overcome these limitations and enable automated QSM processing. Moreover, this method is integrated within an open-source software framework: QSMxT.
Methods
A robust masking technique that automatically separates reliable from less reliable phase regions was developed and combined with a two-pass reconstruction procedure that operates on the separated sources before combination, extracting more information and suppressing streaking artifacts.
Results
Compared with standard masking and reconstruction procedures, the two-pass inversion reduces streaking artifacts caused by unreliable phase and high dynamic ranges of susceptibility sources. It is also robust across a range of acquisitions at 3 T in volunteers and phantoms, at 7 T in tumor patients, and in an in silico head phantom, with significant artifact and error reductions, greater anatomical detail, and minimal parameter tuning.
Conclusion
The two-pass masking and reconstruction procedure separates reliable from less reliable phase regions, enabling a more accurate QSM reconstruction that mitigates artifacts, operates without anatomical priors, and requires minimal parameter tuning. The technique and its integration within QSMxT makes QSM processing more accessible and robust to streaking artifacts.



